You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Dental caries is the most prevalent disease in humans.1 It affects nearly 100% of adults during their lifetime. However, even with this exceptionally high prevalence, the disease is rarely treated. Most procedural efforts in the profession are directed at treating the results of the caries, not the disease. Restorative dentists, most prosthodontists, and many other dental professionals are involved in the treatment of the results of dental caries.
History of Caries Management
Caries treatment has historically been limited to surgical restorative intervention. This is because clinical caries detection tools generally have not had the level of sensitivity needed to identify a caries lesion at a stage early enough to make medicinal therapeutic treatment a viable option. Although nonsurgical interventions have been well established in vitro, they have not been routinely used clinically because early detection methods lacked the effectiveness and reliability to make them feasible.2
To help reduce the risk of caries within a practice, practitioners generally have provided empirical standardized recommendations, including regular brushing, flossing, and use of fluoride toothpaste, for prevention of dental caries. Although these interventions are extremely effective within at-risk populations, they do not necessarily benefit patients at greater-than-average risk. In the future, new approaches and technologies will dramatically change how we think about and treat dental caries. Soon the norm might include mitigating risk to manage the disease process rather than identifying the disease at a later stage that requires surgical restorative intervention.
The Caries Management Continuum
In thinking about the management of dental caries in various phases, one could consider a caries management continuum (Figure 1). In this continuum, the left side reflects the least invasive forms of caries management used prior to the use of surgical restorative interventions. Moving from left to right, interventions progress from salivary and preventive protocols that mitigate risk to minimally invasive restorative dentistry to the latest phases of treatment in the form of traditional operative dentistry. Superimposed on this continuum is the “level of invasivity,” or how aggressive the treatment is in terms of irreversibility. Microbial control, salivary control, remineralization, and resin infiltration are reflective of various treatments one could employ while avoiding surgical intervention. Given the ever-increasing availability of early caries detection tools, and more specific caries risk assessment tools, it will become increasingly possible to deploy nonsurgical approaches in the form of medicinal therapeutic interventions and avoid surgical restorative intervention.
Sensitivity and Specificity
A tool or device exhibits sensitivity if it is able to identify the presence of an existing condition.3 Sensitivity aids in the prevention of false negatives. By this measure, all of the tools that have historically been available for caries detection are extremely insensitive, including visual examination, radiography, and unaided transillumination. A tool or device exhibits specificity if it is able to be accurate in its identification of a condition when it detects said condition.3 In other words, it helps to prevent false positives by not identifying a given disease in a healthy subject.
There are two important practical considerations for clinicians regarding specificity. First, is the detected disease what one thinks it is? For example, if one detects a suspected caries lesion in a visual examination, how certain can one be that it is in fact a caries lesion? Second, will the detected lesion progress if untreated? This question will become even more important as technologies allow very early detection of caries lesions. The earlier caries lesions are detected, the greater the potential risk for over-treatment. In many cases, the natural compensatory remineralization process allows routine “reversal” of very small lesions.4 Despite this, most experts in the subject of early caries lesion detection employ remineralization4 or other intervention techniques for early-detected small lesions, even at the risk of treating some that would not have progressed. An even greater concern is treatment of these early lesions via surgical restorative interventions when they might be treatable in the future with less invasive approaches, or perhaps might not require treatment at all.
Detection and Diagnosis
Caries management consists of two distinct processes: detection and diagnosis of individual caries lesions and patient evaluation to assess risk of caries. Detection and diagnosis occurs through use of visual examination, radiography, or other means and includes charting lesions to be included in a treatment plan for restorative intervention. Detection and diagnosis are not synonymous, and the distinction between them is important. With the development of new technologies that “detect” caries lesions at earlier stages or assist in seeing caries lesions at currently detectable stages, it must be emphasized that these devices do not diagnose. Technologies can be helpful in assisting as one of many sources of information; however, only clinicians can make a diagnosis based on all available data.
Caries detection devices most commonly used today are extremely insensitive. Visual examination using an explorer and/or mirror usually can identify caries lesions only when restorative intervention is needed. The exception to this is the diagnosis of deep pits and fissures needing sealants. Radiography is also extremely insensitive. One can only see caries lesions interproximally on bitewing radiographs when they are halfway through the enamel histologically. This limitation will result in us missing many lesions at the earliest stages when remineralization or other techniques might be effective.
Optical diagnostic technologies have also been developed to assist in the detection of early caries lesions. These can be classified into several subcategories, including those that look for chemical signatures of the biofilm that initiated the caries lesion, those that detect auto fluorescence or loss of fluorescence within the biofilm or caries lesion, those that give a topographical analysis of the lesion, and some that deploy photothermal imaging of the surface. Transillumination to identify caries lesions in anterior teeth has been used for a long time; however, such detection is limited to identifying lesions that are already extensive in their progress through the enamel on the way to cavitation, if not already cavitated.5
Risk Assessment
Risk assessment is a much more complex process than detection and diagnosis, but it is critically important. Only a small percentage of children are classified in the highest risk group for significant caries problems, but it is important that these patients are identified at a very early stage.6 Careful management of the “caries balance” in these patients early on means the progression of a potentially devastating condition can be halted.7 The tools currently available to assess the risk for dental caries can be grouped into two distinct types.
History and Environmental Tools
A history of caries is the greatest risk factor for the development of caries lesions in the future. Even when a patient has a history of a single surface caries lesion, his or her risk for future caries increases dramatically. A family history of caries, especially in the mother, will also increase a child’s caries risk.
A risk assessment tool developed by Featherstone7 and others8 helps practitioners gather this type of data for adults and children. In addition to personal and family history, it also records environmental factors and data such as bacterial counts and salivary flow rates. In addition, the American Academy of Pediatric Dentistry (AAPD) developed a caries assessment tool, or CAT,9 that allows the clinician to determine a child’s relative risk based on collected historical and environmental data. Although CAT is a very useful tool, it requires several minutes of chairside time to gather the required data.
Technology-Based Assessment
Technology-based tools assess specified outcome measures as validated determinants of risk. There are several promising technologies on the market or on the horizon. This includes tools that measure potential acid production in the oral environment. When challenged with sucrose, biofilm that produces acid will allow the caries process to progress, regardless of the quantity or strain of organisms the biofilm contains. Therefore, any diagnostic device that can assess the acid production potential of biofilm could be useful for all patients.
Cariostat (DENTSPLY Sankin, www.dentsply-sankin.com), a test kit available in Japan but not in the United States, is one such tool. Shimono and colleagues at Okayama University found10,11 that Cariostat can reliably predict caries risk as measured by the decayed and filled surfaces (df) outcome measure. The Cariostat test has reliably predicted the short-term caries experience of toddlers as well as long-term df outcomes of children at age 10 years who were sampled as early as age 3. The researchers found that aggressive intervention within a Cariostat elicited high-risk group can prevent the subsequent caries experience. As long as the results are proven and validated, this and other tools assessing acid production potential have possible applications outside the dental office as well.
Caries Management with Interventions
Once risk is assessed and/or disease is identified, what comes next?
Many interventions for treatment exist today, and many more are under development.12 Often these products include various forms of fluoride. Fluoride varnish may be the precursor to other varnish types formulated with agents instead of, or in addition to, fluoride.13 When caries lesions and/or caries risk are detected at the earliest stages, dental professionals can more effectively empower families to become partners in the process of managing their children’s oral health.14,15
Disrupting Biofilm
The bacteria in the mouth that historically were referred to as plaque, we now refer to as part of a dental biofilm. These two terms are not exactly the same. Plaque implies sort of a two-dimensional object that has no form other than the individual bacteria. However, when one talks about a biofilm, one is discussing a community in which bacteria reside in kind of a matrix that supports their growth and also supports the elimination of waste products produced by these bacteria. In the mouth there are as many as 500 to 1,000 different bacteria that interact with one another in a similar way that organs in the human body interact.16 There are signals sent within biofilm wherein one bacterial species in one section of the biofilm might send a signal to another species in a different area, and these signals control the acid production within the biofilm in the presence of fermentable carbohydrates.
Work is only recently emerging to explain the nature of this signaling and how certain chemical compounds and physical interfaces within the biofilm may affect these signals. Various attempts have been made to interfere with the biofilm to mitigate the expression of caries as a disease.12
McLean and others have identified some of the sequencing of protein production that occurs within various species in the biofilm at specific stages after sugar challenge and initiation of pH drop and recovery.17 Specific treatments in the future may halt progression of both small and large caries lesions by extinguishing access to nutrient supply within the biofilm causing the lesion. Although there has been much under development in these areas, there is little to report yet in terms of actual effective treatments related to global microbial control that are readily available, effective, and safe to use in clinical practice.
Saliva Implications
It has been known for years that saliva impairs or enhances the progression of dental caries depending on its level and function. When conditions exist where the salivary production in total is significantly reduced, such as with radiotherapy to the head and neck, there is such a limited production of saliva that dental caries lesions progress extensively and rapidly.18 With salivary deprivation, saliva substitutes can be used. In the future, there may be treatments that target the increase in salivary output in a state of otherwise deficit.
Resin Infiltration
Use of resin infiltration by way of the product Icon® (DMG America, Inc., www.dmg-america.com), has been reported and studied extensively. Although there is not yet widespread adoption of this relatively new technique in clinical practice, the science behind it is strong.19 The technique requires very specific isolation methods and follows a very specific protocol. Work has shown that when following this protocol with careful clinical technique, the likelihood that small early caries lesions interproximally will progress towards the dentinoenamel junction and beyond is substantially reduced.20
Silver Diamine Fluoride
In 2015, the product silver diamine fluoride (SDF) was introduced into the marketplace in the United States (Advantage Arrest, Elevate Oral Care, www.elevateoralcare.com). This product, which has been available for decades in other countries, is an extension in development from silver nitrate, which has been available for more than a century.
SDF is becoming a mainstay in medical management of dental caries today and going forward. The science is strong, and clinicians who have used the product report immediate effect on both small and large caries lesions.21-28 The silver ion within SDF destroys the effect of the biofilm by destroying the vitality of the biofilm itself.29-31 The protocol for use of SDF has been studied extensively and the results, including a recent paper from Horst and colleagues describing the protocol for use over diming fluoride,31 are impressive.
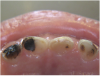
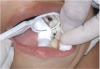
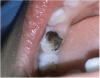
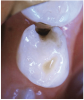
The available SDF product is a clear liquid that is carefully applied in small quantities to the accessible caries lesion. After 30 to 60 seconds of application, the lesion turns black in color, and as reported by each clinician who uses the product, there exists a colorometric indication of biofilm disruption/destruction32,33 (Figure 2). When the patient returns for follow-up after placement of the SDF treatment, it can be explained that the black color indicates biofilm destruction within the lesion and halting of progression of the lesion (Figure 3 and Figure 4). The surface of the dentin below the previously softened caries-affected dentin can be hardened and no longer affected by the biofilm. In some cases, lesions adjacent to the caries lesion intended to be treated can be halted in their earlier stages of development, such as with adjacent proximal lesions (Figure 5).
Early in 2016, an ADA CDT procedure code will be available to code for medical management of dental caries, including that deployed with SDF.
Conclusion
Caries is a disease that manifests itself when the right set of circumstances exists. One needs to have the “right” biofilm, the “right (or wrong)” composition of saliva, and the “right” kind of diet. When these conditions, which should be described as inappropriate conditions, exist, a perfect storm can be created wherein the teeth are decayed very quickly, such as the case in early childhood caries or in rampant caries in adults. In the past, patients have been essentially all the same in terms of how they’ve been treated. In the present and in the future, more specific attention will be given to individual risk factors, biofilms, and salivary compositions; recommendations will be made to patients based on these distinct presentations. More and more, medicinal treatments that do not require restorative surgical intervention will be possible.
Author Information
Joel H. Berg, DDS, MS, is professor and dean of the University of Washington School of Dentistry. Dr. Berg is the author of a number of manuscripts, abstracts, and book chapters on various oral health care subjects, including restorative materials for children and other work related to biomaterials. His current research interests include the development of dental caries prevention programs using risk assessment models and early childhood oral health.
Disclosure
Joel H. Berg, DDS, MS, has received grant/research support from NuSmile, Ivoclar Vivadent, and DMG America.
References
1. Dental caries (tooth decay) in children (age 2 to 11). National Institute of Dental and Craniofacial Research website. www.nidcr.nih.gov/datastatistics/finddatabytopic/dentalcaries. Accessed December 22, 2015.
2. Quock RL, Patel SA, Falcao FA, Barros JA. Is a drill-less dental filling possible? Med Hypotheses. 2011;77(3):315-317.
3. Anvari A, Halpern EF, Samir AE. Statistics 101 for radiologists. Radiographics. 2015;35(6):1789-1801. doi: 10.1148/rg.2015150112.
4. Gluzman R, Katz RV, Frey BJ, McGowan R. Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dentist. 2013;33(3):133-140.
5. Simon JC, Lucas SA, Staninec M, et al. Transillumination and reflectance probes for in vivo near-IR imaging of dental caries. Proc SPIE Int Soc Opt Eng. 2014;8929:89290D. doi: 10.1117/12.2045630.
6. Edelstein BL, Chinn CH. Update on disparities in oral health and access to dental care for America’s children. Acad Pediatr. 2009;9(6):415-419.
7. Featherstone JD. The caries balance: contributing factors and early detection. J Calif Dent Assoc. 2003;31(2):129-133.
8. Cheng J, Chaffee BW, Cheng NF, et al. Understanding treatment effect mechanisms of the CAMBRA randomized trial in reducing caries increment. J Dent Res. 2015;94(1):44-51.
9. American Academy on Pediatric Dentistry Council on Clinical Affairs. Policy on use of a caries-risk assessment tool (CAT) for infants, children, and adolescents. Pediatr Dent. 2008-2009;30(7 suppl):29-33.
10. Nishimura M, Oda T, Kariya N, et al. Using a caries activity test to predict caries risk in early childhood. J Am Dent Assoc. 2008;139(1):63-71.
11. Clarke P, Fraser-Lee NJ, Shimono T. Identifying risk factors for predicting caries in school-aged children using dental health information collected at preschool age. ASDC J Dent Child. 2001;68(5-6):373-378.
12. Klein U, Kanellis MJ, Drake D. Effects of four anticaries agents on lesion depth progression in an in vitro caries model. Pediatr Dent. 1999;21(3):176-180.
13. Chu CH, Lo EC. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36(6):387-391.
14. Mattos-Silveira J, Floriano I, Ferreira FR, et al. Children’s discomfort may vary among different treatments for initial approximal caries lesions: preliminary findings of a randomized controlled clinical trial. Int J Paediatr Dent. 2015;25(4):300-304.
15. Lo EC, Chu CH, Lin HC. A community-based caries control program for pre-school children using topical fluorides: 18-month results. J Dent Res. 2001;80(12):2071-2074.
16. Chu CH, Mei L, Seneviratne CJ, Lo EC. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22(1):2-10.
17. McLean JS, Fansler SJ, Majors PD, et al. Identifying low pH active and lactate-utilizing taxa within oral microbiome communities from healthy children using stable isotope probing techniques. PLoS One. 2012;7(3):e32219. doi: 10.1371/journal.pone.0032219.
18. de Barros da Cunha SR, Ramos PA, Nesrallah AC, et al. The effects of ionizing radiation on the oral cavity. J Contemp Dent Pract. 2015;16(8):679-687.
19. Paris S, Hopfenmuller W, Meyer-Lueckel H. Resin infiltration of caries lesions: an efficacy randomized trial. J Dent Res. 2010;89(8):823-826.
20. Doméjean S, Ducamp R, Léger S, Holmgren C. Resin infiltration of non-cavitated caries lesions: a systematic review. Med Princ Pract. 2015;24(3):216-221.
21. Hamama HH, Yiu CK, Burrow MF. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust Dent J. 2015;60(1):80-87.
22. Mattos-Silveira J, Floriano I, Ferreira FR, et al. New proposal of silver diamine fluoride use in arresting approximal caries: study protocol for a randomized controlled trial. Trials. 2014;15:448. doi: 10.1186/1745-6215-15-448.
23. Mei ML, Chu CH, Low KH, et al. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18(6):e824-e831
24. Beltrán-Aguilar ED. Silver diamine fluoride (SDF) may be better than fluoride varnish and no treatment in arresting and preventing cavitated carious lesions. J Evid Based Dent Pract. 2010;10(2):122-124.
25. Milgrom P, Zero DT, Tanzer JM. An examination of the advances in science and technology of prevention of tooth decay in young children since the Surgeon General’s Report on Oral Health. Acad Pediatr. 2009;9(6):404-409.
26. Yee R, Holmgren C, Mulder J, et al. Efficacy of silver diamine fluoride for Arresting Caries Treatment. J Dent Res. 2009;88(7):644-647.
27. Knight GM, McIntyre JM, Craig GG, et al. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40(2):155-161.
28. Braga MM, Mendes FM, De Benedetto MS, Imparato JC. Effect of silver diammine fluoride on incipient caries lesions in erupting permanent first molars: a pilot study. J Dent Child (Chic). 2009;76(1):28-33.
29. Chu CH, Lo EC. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36(6):387-391.
30. Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84(8):721-724.
31. Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Cal Dent Assoc. 2016;44:17-28.
32. Savas S, Kucukyılmaz E, U Celik E, Ates M. Effects of different antibacterial agents on enamel in a biofilm caries model. J Oral Sci. 2015;57(4):367-372.
33. Mattos-Silveira J, Floriano I, Ferreira FR, et al. New proposal of silver diamine fluoride use in arresting approximal caries: study protocol for a randomized controlled trial. Trials. 2014;15:448.