You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Understanding their efficacy related to apnea severity.
Obstructive sleep apnea (OSA), a sleep-related breathing disorder characterized by intermittent and repetitive cessations in breathing during sleep, affects 3% to 7% of men and 2% to 5% of women in the general adult population.1 However, it is believed to be largely underdiagnosed with one population study indicating that 93% of the female participants and 82% of the male participants with moderate to severe OSA had not been clinically diagnosed despite access to healthcare.2
The disorder has many comorbidities, including type 2 diabetes,3 cardiovascular disease,4 and depression,5 and its impact on society is far-reaching. It is estimated that untreated OSA adds approximately $3.4 billion annually to healthcare costs in the United States.6 In addition, daytime somnolence, a primary symptom of sleep apnea, contributes to the high number of drowsy driving accidents in the United States. Approximately 1,400 lives are lost annually because of OSA-related collisions alone, costing an estimated $15.9 billion dollars.7 In contrast, encouraging early OSA treatment could save an estimated 980 lives and $11.1 billion in collision costs.7 Unfortunately, the longitudinal association between weight change and sleep-disordered breathing8 and the country's ongoing obesity epidemic suggest that OSA will only increase in prevalence, solidifying the condition's status as a public health concern.
To promote more widespread knowledge of OSA among dental healthcare professionals and further the disorder's diagnosis and treatment, this article will explore the various levels of sleep apnea severity (ie, mild, moderate, severe), the available treatment options, and the relevance of these treatment options as they pertain to apnea severity.
An Overview of OSA Severity
OSA severity is primarily determined by an individual's score on the Apnea-Hypopnea Index (AHI), which refers to the number of breathing cessations experienced per hour of sleep (Table 1). Less than 5 apneas per hour denotes normal breathing, 5 to 15 apneas per hour denotes mild sleep apnea, 15 to 30 apneas per hour denotes moderate sleep apnea, and more than 30 apneas per hour denotes severe sleep apnea. It is estimated that as many as 1 out of every 5 adults has at least mild symptoms of OSA whereas about 1 out of every 15 adults has moderate to severe symptoms.9
In combination with AHI score, OSA severity may also be evaluated by taking into account a patient's subjective score on the Epworth Sleepiness Scale (ESS), which is a scale that measures excessive daytime sleepiness. Patients are asked to rate their chance of falling asleep from 0 (would never doze off) to 3 (high chance of dozing off) during a list of activities that require varying degrees of attention. These numbers are added together to determine the patient's ESS score (Table 2). Using the ESS as a guide, individuals with mild OSA experience involuntary sleepiness at times of activity that demand little attention, such as while watching television or reading; those with moderate OSA experience involuntary sleepiness during activities that require some attention, such as during meetings or presentations; and those with severe OSA experience involuntary sleepiness during activities that require much attention, such as while talking or driving.
Finally, OSA severity may also be evaluated by taking into account the patient's oxygen desaturation index, or ODI. ODI is a measure of the number of times that the patient's oxygen level drops by 4% or more per hour of sleep. Although an ESS score and ODI score alone cannot be used to diagnose OSA, when coupled with a problematic AHI score, desaturation events and/or a high ESS score can help to confirm an OSA diagnosis and subsequently determine its severity.
The symptoms of OSA are experienced with an increasing intensity that is dependent on the severity of the condition. Chronic loud snoring and excessive daytime sleepiness, which are experienced by more than 80% of diagnosed patients,10 are the most common symptoms of OSA. Other symptoms of OSA include headaches; cognitive impairment affecting working memory, attention, and executive function; and mood disturbances, particularly anxiety and depression. These symptoms can be relieved through treatment.
Treatment Options
The three most common methods of treating sleep apnea are continuous positive airway pressure (CPAP) therapy, oral appliance therapy, and surgery. Because obesity is a key risk factor for OSA, weight loss is also considered a viable treatment option. A less common treatment option is supine-avoidance therapy, in which patients abstain or are prevented from sleeping in the supine position. In addition, if satisfactory progress in the amelioration of symptoms has not been gained with any individual treatment option, combination therapy may offer a suitable alternative. Regardless of the options pursued, OSA treatment may be considered successful when the patient demonstrates an AHI score of < 5.
CPAP Therapy
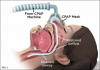
CPAP therapy is recognized as the mainstay treatment option for OSA patients and is effective in treating mild, moderate, and severe sleep apnea. CPAP machines use a pump to apply mild air pressure on a continuous basis, opening up the patient's airway and preventing the soft tissue from collapsing (Figure 1).11 Although CPAP has been proven efficacious for the treatment of OSA and successful in mitigating its symptoms, including high blood pressure,12 with CPAP adherence rates ranging from 30% to 60%,13 poor adherence to the therapy is widely recognized as a significant limiting factor. As a result, a number of patients ultimately abandon or continue to inadequately use CPAP as a means of treating OSA.
Other positive airway pressure devices available to patients include bi-level positive airway pressure (BPAP) machines and autotitrating positive airway pressure (APAP) machines. Although similar to CPAP machines, BPAP and APAP machines offer unique features that benefit some patients.
Unlike traditional CPAP machines that provide the same level of pressure between cycles, the BPAP machine administers varying levels of air pressure between the inspiratory and expiratory cycles. The shifting pressure of the BPAP machine from the inspiratory cycle to the expiratory cycle may be guided by the spontaneous breathing of the patient or set by a respiratory rate programmed into the machine. BPAP machines facilitate a tolerance to higher pressures and can be particularly efficacious for patients with low oxygen levels and those who do not respond to CPAP therapy. In addition, BPAP machines can generate air pressures above the maximum CPAP level of 20 cmH2O, making them valuable for patients with the most severe cases of OSA. In a clinical trial comparing the adherence rates of BPAP therapy and CPAP therapy, the rate of adherence to BPAP therapy was 10 points higher.14
APAP machines automatically adjust to the patient's changing breathing patterns throughout the night. OSA treatment with APAP therapy results in nearly identical outcomes to CPAP therapy with similar rates of adherence, reductions in AHI score, and improvements in daytime sleepiness.15,16 Of note, CPAP therapy is more favorable regarding positive blood pressure outcomes, perhaps due to the relatively lower average air pressure generated by APAP machines when compared with CPAP machines.15
Oral Appliance Therapy
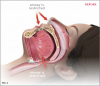
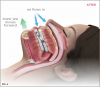
Oral appliance therapy for OSA falls into two broad categories: mandibular advancement devices (MADs) (Figure 2), which include splints, and tongue repositioning or retaining devices (TRDs). During sleep, MADs move the mandible forward to open the airway and prevent collapse (Figure 3 and Figure 4), and in a similar fashion, TRDs move the tongue forward to prevent it from obstructing the airway. Oral appliances are generally less efficacious in reducing a patient's AHI score than CPAP therapy; however, they have been shown to achieve reduced cardiovascular mortality rates that were similar to those achieved by CPAP therapy17 and have a self-reported median compliance rate of 77% of nights.18 The superiority of CPAP therapy in reducing AHI score may be lessened by patients' low rate of adherence, resulting in similar degrees of effectiveness for both CPAP therapy and oral appliance therapy in clinical practice.19
MADs are the most common type of oral appliance, and they are recommended as a first-line treatment for mild to moderate OSA; moreover, they provide a suitable alternative for patients with severe OSA who are unable to tolerate CPAP therapy.19,20 Through the use of MADs, complete resolution of OSA (ie, AHI score reduced to < 5 apneas per hour) occurs in approximately 40% of patients.19 Many factors have been associated with success in MAD treatment, including female gender, younger age, supine-dependent OSA, lower BMI, smaller neck circumference, and craniofacial factors; however, a reliable method for predicting success in the clinical setting has yet to be established.19
Most complications of MAD treatment are mild and temporary and occur during the short-term as patients acclimate to the device. They include hypersalivation, dry mouth, dental pain, gingival irritation, myofascial pain, and temporomandibular joint discomfort. Long-term adverse reactions to MAD treatment include changes to the craniofacial structure, which are largely subclinical but can present a problem for a small subset of patients.19
TRDs perform similarly to MADs, and as such, they are considered efficacious for the treatment of mild to moderate OSA and an acceptable alternative for patients with severe sleep apnea who are intolerant to CPAP therapy. TRDs have been shown to produce significant improvement in severe sleep apnea cases. In one study, TRD treatment reduced AHI score from 38 to 14 and decreased subjective snoring intensity by 68%.21
Surgery
Surgical treatments for OSA aim to improve the patency of the airway by addressing sites of obstruction. Standard OSA surgery options include nasal surgery, uvulopalatopharyngoplasty, maxillomandibular advancement surgery, tongue reduction surgery, craniofacial surgery, and in highly selected patients, tracheostomy. Surgery is best reserved for severe cases in which the patient has identifiable anatomical problems (eg, enlarged tonsils) or for cases in which neither CPAP therapy nor oral appliance therapy is a viable option. In addition, because no single surgical option will suit all patients, surgery should be considered on a patient-by-patient basis and only undertaken after comprehensive patient counseling has occurred. Cure rates with surgery vary, but overall, surgery has been proven effective in decreasing the morbidity and mortality associated with OSA.22
Weight Loss/Lifestyle Changes
Weight loss can reduce OSA severity while mitigating the cardiometabolic consequences that are related to both OSA and obesity. Studies have shown that a weight loss of 10% is associated with a 26% reduction in AHI score23; therefore, weight loss benefits OSA patients, regardless of their level of severity, and it can be achieved through dietary modifications, exercise, and surgical interventions. In particular, the performance of bariatric surgery results in markedly greater improvement in body mass index and AHI score when compared with nonsurgical alternatives.24
Supine-Avoidance Therapy
More than 50% of all OSA patients experience a worsening of their condition in the supine position.25 Furthermore, in one study, more than one-third of the observed patients with severe OSA were positional patients.26 In supine-avoidance or positional therapy, a device is used to provide a subtle vibrating stimulus that stops the patient from adopting the supine position. Although less researched than other OSA treatment methods, there is strong evidence that this new generation of devices for positional therapy is effective in reducing patients' AHI scores during short-term follow-up.27 Additional long-term studies are needed; however, the use of positional therapy in combination with other treatment methods may prove useful to target individual patient traits.
Combination Therapy
As the name suggests, combination therapy involves the combination of multiple OSA treatment options to target individual patient traits, and it may be a suitable alternative for cases in which positive treatment outcomes have not been achieved by either oral appliance therapy or CPAP therapy alone. In one study, patients with severe OSA who were intolerant to positive airway pressure therapy and unresponsive to MAD treatment experienced positive outcomes with combination therapy, which showed additive effects in reducing AHI and ODI scores.28 In addition, for patients who exhibited residual supine-dependent OSA with MAD treatment, combining MAD treatment with supine-avoidance therapy led to higher therapeutic efficacy than the use of either treatment modality alone.29
Discussion
OSA is a serious condition that, when left untreated, results in numerous comorbidities and has a far-reaching impact on society. Patients present with varying degrees of apnea severity, but fortunately, a multitude of effective treatment options are available. By increasing awareness regarding the prevalence of OSA and becoming educated about the available therapies, clinicians can contribute to helping a greater number of patients achieve a higher quality of life through the provision of efficacious OSA treatment.
Queries regarding this course may be submitted to authorqueries@aegiscomm.com
About the Author
Jeff Rodgers, DMD Diplomate
American Board of Dental Sleep Medicine
Diplomate
American Sleep and Breathing Academy
Private Practice
Atlanta, Georgia
References
1. Lurie A. Obstructive sleep apnea in adults: epidemiology, clinical presentation, and treatment options. Adv Cardiol. 2011;46:1-42.
2. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705-706.
3. Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. eCollection 2012.
4. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19-25.
5. Shoib S, Malik JA, Masoodi S. Depression as a manifestation of obstructive sleep apnea. J Neurosci Rural Pract. 2017;8(3):346-351.
6. Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749-755.
7. Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(23):1908-1913.
8. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015-3021.
9. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
10. Spicuzza L, Caruso D, Di Maria G. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis. 2015;6(5):273-285.
11. Watson S. Weight loss, breathing devices still best for treating obstructive sleep apnea. Harvard Health Publishing website. https://www.health.harvard.edu/blog/weight-loss-breathing-devices-still-best-for-treating-obstructive-sleep-apnea-201310026713. Updated March 18, 2019. Accessed February 25, 2020.
12. Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107(1):68-73.
13. Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245-258.
14. Atwood CW Jr. Progress toward a clearer understanding of the role of bilevel positive airway pressure therapy for obstructive sleep apnea. J Clin Sleep Med. 2013;9(4):337-338.
15. Liu T, Li W, Zhou H, Wang Z. Verifying the relative efficacy between continuous positive airway pressure therapy and its alternatives for obstructive sleep apnea: a network meta-analysis. Front Neurol. 2017;8:289. doi: 10.3389/fneur.2017.00289.
16. Berry RB, Sriram P. Auto-adjusting positive airway pressure treatment for sleep apnea diagnosed by home sleep testing. J Clin Sleep Med. 2014;10(12):1269-1275.
17. Anandam A, Patil M, Akinnusi M, et al. Cardio-vascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: an observational study Respirology. 2013;18(8):1184-1190.
18. Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
19. Sutherland K, Cistulli P. Mandibular advancement splints for the treatment of sleep apnea syndrome. Swiss Med Wkly. 2011;141:w13276. doi: 10.4414/smw.2011.13276.
20. Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499-521.
21. Lazard DS, Blumen M, Lévy P, et al. The tongue-retaining device: efficacy and side effects in obstructive sleep apnea syndrome. J Clin Sleep Med. 2009;5(5):431-438.
22. Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics. 2012;9(4):710-716.
23. Motamedi KK, McClary AC, Amedee RG. Obstructive sleep apnea: a growing problem. Ochsner J. 2009;9(3):149-153.
24. Ashrafian H, Toma T, Rowland SP, et al. Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obes Surg. 2015;25(7):1239-1250.
25. Omobomi O, Quan SF. Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath. 2018;22(2):297-304.
26. Oksenberg A, Gadoth N, Töyräs J, Leppänen T. Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA. Sleep Breath. 2019. doi: 10.1007/s11325-019-01897-1.
27. Ravesloot MJL, White D, Heinzer R, et al. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13(6):813-824.
28. Liu HW, Chen YJ, Lai YC, et al. Correction: Combining MAD and CPAP as an effective strategy for treating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS One. 2018;13(4):e0196319. doi: 10.1371/journal.pone.0196319.
29. Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19(2):637-644.