You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
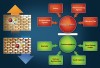
The oral cavity is a battlefield of activities of demineralization and remineralization (Figure 1). The ratio between demineralization and remineralization is crucial, determining the hardness and strength of tooth structure. Demineralization results from a complex chemistry between bacteria, diet, and salivary components. A drop in the pH in the oral cavity results in demineralization, and the oral environment becomes undersaturated with mineral ions, relative to a tooth’s mineral content. A drop in the pH is due to the organic acids (ie, lactic acid) that are produced by the action of plaque bacteria in the presence of dietary carbohydrates. If the demineralization phase continues for a long period of time, excessive loss of minerals results, which leads to loss of enamel structure and cavitation—the typical characteristics of dental caries. This dissolution continues until the pH returns to normal level.
When the pH rises, the reverse takes place, resulting in deposition of mineral back to the tooth structure.1 Thus, conversely, remineralization occurs when pH rises and there is deposition of calcium, phosphate, and fluoride ions in the form of fluorapatite, which are more resistant to crystals’ dissolution by organic acids.2 During remineralization, growth of newly formed crystals (fluorapatite) takes place, and, with advancing growth, the crystals fuse with each other to form large crystals with hexagonal outlines.3 Therefore, the best strategy for caries management is to focus on the methods of improving the remineralizing process with the aid of remineralization products. Contemporarily, a variety of remineralizing agents like fluorides, casein calcium phosphopeptides, NovaMin®, etc, that aid in remineralization of tooth structure are available commercially (Table 1, Table 2, Table 3, Table 4 and Table 5). This article provides an overview of the various available agents and their roles in the remineralization of tooth tissue as well as their dispensing methods and clinical implications.
Remineralizing Agents
Fluorides
Fluorides are an important adjunct in the prevention of dental caries. They are introduced into the oral environment via personal (eg, dentifrices, rinses) or professional applications (eg, varnishes, foams, gels, fluoride-releasing restorative materials). Fluoride levels of about 3 parts per million (ppm) in the enamel are required to shift the balance from net demineralization to net remineralization.4 Several mechanisms have been suggested to achieve the anticaries effects of fluoride, including the formation of fluorapatite, which is more acid-resistant than hydroxyapatite; the enhancement of remineralization; interference of ionic bonding during pellicle and plaque formation; and the inhibition of microbial growth and metabolism.5 Fluoride can be used in combination with sodium, tin, or titanium. The newly introduced titanium fluoride (TiF) exhibits enhanced uptake of calcium, and TiF-pretreated enamel also shows less loss of calcium during demineralization.6
Casein Phosphopeptides (CPP)
Casein phosphopeptides are the latest entry into preventive dentistry. They are used alone or as CPP-ACP (casein phophopeptides with amorphous calcium phosphate) or CPP-ACFP (casein phophopeptides with amorphous calcium fluoride phosphate). CPP-ACP has shown to reduce demineralization and enhance remineralization of the enamel subsurface carious lesions.7 The main function of casein phosphopeptides is to modulate bioavailability of calcium phosphate levels by maintaining ionic phosphate and calcium supersaturation to increase remineralization.8,9 The role of ACP is also said to control the precipitation of CPP with calcium and phosphate ions.10 The advantage of CPP-ACFP is the availability of calcium, phosphate, and fluoride in one product.7 Each molecule of CPP can bind up to 25 calcium ions, 15 phosphate ions, and 5 fluoride ions. The calcium phosphate in these complexes is biologically available for remineralization of subsurface lesions in tooth enamel.10,11
CPP also is believed to have an antibacterial and buffering effect on plaque and interfere in the growth and adherence of Streptococcus mutans and Streptococcus sorbinus. It has been observed that CPP-ACP significantly reduced caries activity in a dose-dependent manner, as 1% CPP-ACP produced about a 55% reduction in smooth surface caries and a 46% reduction in fissure caries activity, which is similar in effect to that produced by 500 ppm of fluoride. Combined with fluoride, CPP-ACP has an additive effect on caries activity.10,12 Use of CPP-ACP along with fluoride-containing dentifrice has proved to be beneficial in reducing the demineralization around orthodontic brackets and remineralizing white spots caused by demineralization.13,14 Ramalingam et al15 found that adding CPP-ACP to soft drinks can reduce their erosion capacity. CPP-ACP has also been added to dentifrices, mouthrinses, chewing gums, lozenges, and bovine milk. A study by Walker et al16 found that although milk contains casein phosphate, addition of CPP-ACP results in enhanced remineralization. A dose of 5 gm of CPP-ACP produced 148% more remineralization compared to 2 gm of CPP-ACP per liter of milk.
Sugar Substitutes
Xylitol is a commonly used sugar substitute, especially in chewing gums. A nonfermentable sugar alcohol acts as a carrier or reservoir for calcium phosphates.17 In a trial, Manton et al18 showed that a sugar-free gum containing xylitol produces superior remineralization. The addition of fluoride to xylitol is said to provide additional benefit, assuming the fluoride concentration is more than 0.8 ppm. Xylitol restrains remineralization when the concentration of available fluoride is low.19 Besides fluoride, calcium lactate also enhances remineralization when added to xylitol.20
Sorbitol is another sugar substitute that is used as an artificial sweetener. The abilities of xylitol and sorbitol to remineralize early enamel caries seem to be almost similar.21 Isomalt is a noncariogenic sweetener that is widely used as a sugar substitute. Adding isomalt to a demineralizing solution has shown to significantly reduce tooth mineral loss.22
Calcium Sodium Phosphosilicate
NovaMin® (calcium sodium phosphosilicate) is the trademark product of NovaMin Technology Inc. (NTI), which was acquired by GlaxoSmithKline in 2009. The compound is a bioactive glass composed of minerals that naturally occur in the body and reacts when it comes into contact with water, saliva, or other body fluids. This reaction releases calcium, phosphorus, sodium, and silicon ions in a way that results in the formation of new hydroxycarbonateapatite (HCA) crystals.23
Ozone
Ozone is a chemical compound consisting of three oxygen atoms (O3, triatomic oxygen). Ozone therapy has proven to be effective with a wide range of dental applications, including prosthodontics, endodontics, periodontics, surgical procedures, and preventive dentistry.24 It is usually advocated in dentistry for sterilization of cavities, root canals, periodontal pockets, and herpetic lesions. Ozone therapy is also proposed to stimulate remineralization of incipient caries following treatment for a period of about 6 to 8 weeks.24,25
Hydroxyapatite
Carbonate hydroxyapatite nanocrystals, having size, morphology, chemical composition, and crystallinity comparable to that of dentin, are said to remineralize enamel.26 A concentration of 10% nanohydroxyapatite is optimal for remineralization of early enamel caries.27 Hydroxyapatite has been used in toothpastes (as fillers) and pit-and-fissure sealants. Hydroxyapapite crystals can effectively penetrate the dentin tubules and obturate them and can cause closure of the tubular openings of the dentin with plugs within 10 minutes as well as a regeneration of a surface mineral layer.26,27
Dispensing Methods
Remineralizing agents can be incorporated into different products for application. Commonly used vehicles are restorative materials, pit-and-fissure sealants, dentifrices, chewing gums, and rinses.
Restorative Materials
Fluoride-releasing materials used in restorative dentistry are glass ionomers, compomers, and giomers.28,29
Glass Ionomers
Fluoride released from a glass-ionomer restoration has been found to be incorporated in adjacent tooth enamel and saliva.30 It has been observed that fluoride released from restorative materials has an effective zone of about 1 mm from the restoration’s margin.31 Glass ionomers also incorporate into bacteria, thus inhibiting bacterial acid production.32,33 Comparing their fluoride-releasing ability, conventional glass ionomers release comparatively large amounts of fluorides compared to resin-modified glass ionomers. Salivary fluoride concentration is found to remain elevated for up to 1 year after placement of glass-ionomer restorations (0.3 ppm after placement and 0.04 ppm 1 year later).31 Perrin et al34 reported that the greatest release of fluoride from glass ionomer occurred on the first day, followed by a sharp decrease on the second day, and gradually diminishing over 3 weeks to a low-level, long-term release. They even observed fluoride release of at least 0.5 ppm even after 1 year. Certain studies reported a “burst” of fluoride release, with high early release for 1 to 2 days, followed by a rapid decline.35-38 Placing an adhesive or protective surface coating on the glass-ionomer restoration as a final step after finishing is a routine practice. This coating may delay the fluoride release and subsequent uptake until abrasion removes the coating. Burkett et al36 and McKnight-Hanes and Whitford39 reported that varnishing glass-ionomer samples decreased fluoride release by 61% to 76%. Amount of fluoride release is also dependent on and directly proportional to the surface area of the restoration.40
Glass ionomer (conventional and resin-modified) and compomers can be recharged from external sources such as topical fluoride application.41-43 Resin-modified and conventional glass ionomers have been found to have similar fluoride release and recharge rates.44 The fluoride release must be maintained to about 2 to 3 µg/mL/day for effective remineralization, and this can be achieved by fluoride recharge.4 Conventional and resin-modified glass ionomers have demonstrated the greatest fluoride recharge capacity; however, fluoride-releasing resin-based composites do not release any additional fluoride after being exposed to a fluoride-rich solution. Compomers have recharge capacity intermediately between resin-modified glass ionomers and resin composites. The recharge ability of these materials may be due to the microporosities present in conventional and resin-modified glass ionomers.4 Topical fluorides form the main source for recharging of these restorative materials, which depends on the pH of the fluoride agent. Acidic topical fluoride solutions found in acidulated phosphate fluoride solutions and other acidified fluoride preparations cause degradation of glass-ionomer materials and should, therefore, be avoided.45,46 Resin-modified glass ionomers are more resistant to surface degradation than conventional glass ionomer but still degrade when exposed to acids.47
Compomers
Compomers contain polyacid-modified monomers with fluoride-releasing silicate glasses and are formulated without water. They are used for restorations in low stress-bearing areas and for patients at medium risk of developing caries, or when using the sandwich technique. Compomers release fluoride by a mechanism similar to that of glass and hybrid ionomers, but the amount of fluoride release and its duration are less than those of glass and hybrid ionomers. Also, compomers do not recharge from fluoride treatments.48
Giomers
Giomer restoratives are a hybridization of glass ionomer and resin composite.49 They have the fluoride-release and recharge properties of glass-ionomer cements along with excellent esthetics, easy polishability, and strength of resin composites. Yap et al50 found that while giomer released fluoride, it did not have an initial “burst” type of release like glass ionomers. Their long-term release of fluoride is lower than that of the other materials.
Pit-and-Fissure Sealants
Pit-and-fissure sealants are materials used in preventive dentistry that provide a mechanical barrier against lodgment of bacteria in deep pits and fissures. Available sealants are either resin-based or glass-ionomer-based. Since resin-based sealants do not provide fluoride release, glass-ionomer sealants are more effective for caries prevention. The addition of remineralizing agents such as fluorides and CCP-ACP can further enhance remineralization (Table 1).
Fluorides are incorporated into the fillers (strontium-fluoride-aluminosilicate glass) of sealants. Fluoride release occurs through hydrolysis and external and internal diffusion.51 The addition of ACP to resin sealants has made them comparable to glass-ionomer-based sealants.52 Meyers and Eanes showed that the solubility of ACP enables it to release supersaturating levels of calcium and phosphate ions in proportion that is favorable for hydroxyapatite formation.53 ACP-containing sealants have a higher remineralizing capacity with a tendency to remineralize enamel subsurface lesions.54,55
Dentifrices
Dentifrices are one of the most practical methods for delivering remineralizing agents. Fluoride is one of the most commonly used remineralizing agents. Achieving densely filled prism cores, the quality of remineralization following the use of fluoride dentifrice was found to be superior compared to fluoride rinse.56 Fluoride in dentifrice may be available as single-fluoride sourced products (sodium fluoride [NaF], stannous fluoride [SnF2], amine fluoride [AmF], or sodium monofluorophosphate [NaMFP]) or dual-fluoride active products (NaF + sodium monofluorophosphate [SMFP], AmF + SnF2) (Table 2). Products containing NaMFP are said to provide the highest level of fluoride compared to others.57 The addition of xylitol (5%) to fluoride-containing (500 ppm) dentifrice is found to enhance remineralization compared to fluoride alone.58 A recent trend is to incorporate CCP-ACP along with fluoride, which is found to have an additive effect. Burwell and Muscle found that CPP-ACP provided sustained condition for remineralization when used in a dentifrice.23 Additionally, the use of 1,500 ppm fluoridated NaF toothpaste has been found to be more effective on the remineralization process than the use of a 50 ppm NaF mouthrinse.56
Chewing Gums
Chewing gums are an effective method for caries prevention. When chewed for long periods they stimulate saliva and have a washing off effect on debris. They also can be used to carry the desired medicaments to the tooth, thus having a multiple benefit. Agents such as fluoride and CPP-ACP are added to improve the anticaries potential of chewing gums (Table 3). Gum arabic, which is the main ingredient of chewing gum, is considered to have an ability to enhance remineralization, probably because of its high concentration of calcium (Ca2+).59 It has been found that chewing gum for about 30 minutes after meals and snacks considerably aided in remineralization.60-62
Xylitol is the most common sweetener to be used as a sugar substitute in chewing gums, followed by sorbitol.63 The addition of CPP-ACP along with xylitol has been found to produce superior remineralization.18 Cai et al64 found that CPP-ACP produced better remineralization even in the presence of citric acid, which means that CPP-ACP can be used to promote remineralization in the presence of an acidic environment. It has also been observed that the effect of xylitol along with calcium lactate improved remineralization.20
Pastes, Rinses, and Dental Floss
Pastes used for remineralization contain calcium- and phosphate-realizing components (eg, CCP-ACP) with or without fluoride. Commercial pastes containing CPP are designed for professional application as well as professionally supervised home application. They can be applied via prophy cup, custom tray, toothbrush, or fingertip. Use of CPP-ACP along with fluoride-containing dentifrice has proved to be beneficial in reducing the demineralization around orthodontic brackets and remineralizing white spots caused by demineralization.11,12 Remineralizing agents such as fluoride, NovaMin, etc, can also be added to the prophylactic paste for additional benefit. Recently, an innovative paste containing a tricalcium phosphate ingredient was introduced that features fluoride along with calcium and phosphate with a protective barrier around the calcium that inhibits its reaction with fluoride ions. As the toothpaste comes in contact with saliva during brushing, the barrier breaks down and makes the calcium, phosphate, and fluoride readily available to the tooth, promoting remineralization.
Mouthrinses (Table 4) are vehicles used to carry various medicaments to the areas of dentition that are difficult to brush. In a study by Reynolds et al, mouthrinse containing CPP-ACP and fluoride has shown to increase fluoride uptake into plaque.65
Dental floss is an important adjunct to deliver fluoride to the interproximal areas of teeth. Studies have shown that fluoride can be released from flossing with SnF2-impregnated dental floss (Table 5), elevating salivary fluoride levels for at least 30 minutes. Thus, use of fluoride-containing dental floss is an alternate option for delivery of fluoride to individuals at risk of dental caries.66
Future Prospects
Tissue engineering is the field of functional restoration of tissue structure and physiology for impaired or damaged tissues due to cancer, diseases, and trauma.67 Contemporarily, this field is also being exploited in dentistry. Regenerative dentistry applies tissue engineering approaches for the repair/regeneration of pulp tissue organ using three basic key elements: an extracellular matrix scaffold (which can be synthetic), progenitor/stem cells, and inductive morphogenetic signals68 that are exposed to a conductive environment to regenerate a vital and functional tissue and/or organ.69,70 Pulp stem/progenitor cells, which have the characteristics of extensive self-renewal capacity and maintenance throughout the life of an organism, can be exploited in culturing large numbers of pulp stem cells in vitro.71 Morphogens are inductive signals that function as growth/differentiation factors in odontoblast differentiation. Recombinant human BMP2, BMP4, and BMP7 have shown to induce reparative/regenerative dentin formation in vivo.72 Scaffolds provide a physicochemical surface for the differentiation of odontoblasts from pulp cells. An ideal method would be to fabricate a material using these three basic elements that would mimic apatite crystals. Recently, nanotechniques, nanoscopic tools, and x-ray scanning tomography (XTM) have been used to create a 3-dimensional profile of normal and carious dentin microarchitecture (l µm x l µm x l µm cube).73 Also, synthetic carbonate-hydroxyapatite biomimetic (CHA) nanocrystals have been investigated regarding the possibility of obtaining an in vitro remineralization of the altered enamel surfaces.74 Treatment of demineralized enamel with CHA nanocrystals for just 10 minutes induced a consistent enamel remineralization through the formation of a surface carbonate-hydroxyapatite coating. This coating is caused by the chemical bond of the synthetic CHA nanocrystals onto the surface prismatic hydroxyapatite enamel.
References
1. Larsen MJ, Pearce EI. Saturation of human saliva with respect to calcium salts. Arch Oral Biol. 2003;48(4):317-322.
2. Margeas R. Remineralization with a unique delivery system. Inside Dentistry. 2006;(2)4:86.
3. Tohda H, Yanagisawa T, Tanaka N, Takuma S. Growth and fusion of apatite crystals in the remineralized enamel. J Elect Micro. 1990;39(4):238-244.
4. Summit JB, Robbins WJ, Schwartz RS. Fundamentals of Operative Dentistry. A Contemporary Approach. 2nd ed. Chicago, IL: Quintessence Publishing; 2001;377-385.
5. Niessen LC, Gibson G. Oral health for a lifetime: preventive strategies for the older adult. Quintessence Int. 1997;28(9):626-630.
6. Exterkate RA, ten Cate JM. Effects of a new titanium fluoride derivative on enamel de- and remineralization. Eur J Oral Sci. 2007;115(2):143-147.
7. Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134(4):989S-995S.
8. Cross KJ, Huq NL, Reynolds EC. Casein phosphopeptides in oral health—chemistry and clinical applications. Curr Pharm Des. 2007;13(8):793-800.
9. Schüpbach P, Neeser JR, Golliard M, et al. Incorporation of
caseinoglycomacropeptide and caseinophosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J Dent Res. 1996;75(10):1779-1788.
10. Cross KJ, Laila Huq N, Palamara JE, et al. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J Biol Chem. 2005;280(15):15362-15369.
11. Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76(9):1587-1595.
12. Reynolds EC, Cain CJ, Webber FL, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res. 1995;74(6):1272-1279.
13. Sudjalim TR, Woods MG, Manton DJ, Reynolds EC. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2007;131(6):705.e1-705.e9.
14. Andersson A, Sköld-Larsson K, Hallgren A, et al. Effect of a dental cream containing amorphous cream phosphate complexes on white spot lesion regression assessed by laser fluorescence. Oral Health Prev Dent. 2007;5(3):229-233.
15. Ramalingam L, Messer LB, Reynolds EC. Adding casein phosphopeptide-amorphous calcium phosphate to sports drinks to eliminate in vitro erosion. Pediatr Dent. 2005;27(1):61-67.
16. Walker G, Cai F, Shen P, et al. Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J Dairy Res. 2006;73(1):74-78.
17. Yoshihiko H, Tsunenori M, Iluminada VL. X-ray microanalysis of remineralized enamel lesions by xylitol-containing chewing gums having different types of calcium phosphate. Japanese J Cons Dent. 2005;48(5):648-655.
18. Manton DJ, Walker GD, Cai F, et al. Remineralization of enamel subsurface lesions in situ by the use of three commercially available sugar-free gums. Int J Paediatr Dent. 2008;18(4):284-290.
19. Toshinari M. Remineralization promoting effect of chewing gum containing fluoride and xylitol. Japanese J Pediatric Dent. 2005;43(1):1-11.
20. Suda R, Suzuki T, Takiguchi R, et al. The effect of adding calcium lactate to xylitol chewing gum on remineralization of enamel lesions. Caries Res. 2006;40(1):43-46.
21. Manning RH, Edgar WM, Agalamanyi EA. Effects of chewing gums sweetened with sorbitol or a sorbitol/xylitol mixture on the remineralisation of human enamel lesions in situ. Caries Res. 1992;26(2):104-109.
22. Takatsuka T, Exterkate RA, ten Cate JM. Effects of Isomalt on enamel de- and remineralization, a combined in vitro pH-cycling model and in situ study. Clin Oral Investig. 2008;12(2):173-177.
23. Burwell AK, Muscle D. Sustained calcium ion and pH release from calcium phosphate-containing dentifrices. Paper presented at: IADR/AADR/CADR 87th General Session and Exhibition; April 3, 2009; Miami, FL.
24. Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008;9(4):75-84.
25. Huth KC, Paschos E, Brand K, Hickel R. Effect of ozone on non-cavitated fissure carious lesions in permanent molars. A controlled prospective clinical study. Am J Dent. 2005;18(4):223-228.
26. Rimondini L, Palazzo B, Iafisco M, et al. The remineralizing effect of carbonate-hydroxyapatite nanocrystals on dentine. Materials Science Forum. 2007;539-543:602-605.
27. Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4(3):34104.
28. Burgess JO, Norling BK, Summitt JB. Advances in glass ionomer material. Esthet Dent Update. 1993;4:54-58.
29. Burgess JO, Norling BK, Rawls HR, Ong JL. Directly placed esthetic restorative materials—the continuum. Compend Cont Educ Dent. 1996;17(8):731-748.
30. Hatibovic-Kofman S, Koch G. Fluoride release from glass ionomer cement in vivo and in vitro. Swed Dent J. 1991;15(6);253-258.
31. Tantbirojn D, Douglas WH, Versluis A. Inhibitive effect of resin-modified glass ionomer cement on remote artificial caries. Caries Res. 1997;31(4):275-280.
32. Benelli EM, Serra MC, Rodrigues AL Jr, Cury JA. In situ anticariogenic potential of glass ionomer cement. Caries Res. 1993;27(4):280-284.
33. Seppa L, Korhonen A, Nuutinen A. Inhibitory effect on S. mutans by fluoride-treated conventional and resin-reinforced glass ionomer cements. Eur J Oral Sci. 1995;103(3):182-185.
34. Perrin C, Persin M, Sarrazin J. A comparison of fluoride release from four glass-ionomer cements. Quintessence Int. 1994;25(9):603-608.
35. Alvarez AN, Burgess JO, Chan DCN. Short-term fluoride release of six ionomers—recharged, coated and abraded. J Dent Res. 1994;73(1 suppl):134.
36. Burkett L, Burgess JO, Chan DC, Norling BK. Fluoride release of glass ionomers coated and not coated with adhesive. J Dent Res. 1993;72(1 suppl):258.
37. Eichmiller FC, Marjenhoff WA. Fluoride-releasing dental restorative materials. Oper Dent. 1998;23(5):218-228.
38. Woolford MJ, Grieve AR. Release of fluoride from glass polyalkenoate (ionomer) cement subjected to radiant heat. J Dent. 1995;23(4):233-237.
39. McKnight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res. 1992;26(5):345-350.
40. Cranfield M, Kuhn A, Winter GB. Factors relating to the rate of fluoride-ion release from glass-ionomer cement. J Dent. 1982;10(4):333-341.
41. Forsten L. Fluoride release and uptake by glass ionomers. Scand J Dent Res. 1991;99(3):241-245.
42. Damen JJ, Buijs MJ, ten Cate JM. Uptake and release of fluoride by saliva-coated glass ionomer cement. Caries Res. 1996;30(6):
454-457.
43. Diaz-Arnold AM, Holmes DC, Wistrom DW, Swift EJ Jr. Short-term fluoride release/uptake of glass ionomer restoratives. Dent Mater. 1995;11(2):96-101.
44. de Araujo FB, Garcia-Godoy F, Cury JA, Conceicao EN. Fluoride release from fluoride-containing materials. Oper Dent. 1996;21(5):185-190.
45. Burgess JO, Cardenas HL. Surface roughness of topical fluoride treated glass ionomers. J Dent Res. 1995;74:108.
46. Diaz-Arnold AM, Wistrom DW, Swift EJ Jr. Topical fluoride and glass ionomer microhardness. Am J Dent. 1995;8(3):134-136.
47. El-Badrawy WA, McComb D. Effect of home-use fluoride gels on resin-modified glass-ionomer cements. Oper Dent. 1998;23(1):2-9.
48. Ruse ND. What is a “compomer”? J Can Dent Assoc. 1999;65(9):500-504.
49. Gordan VV, Mondragon E, Watson RE, et al. A clinical evaluation of a self-etching primer and a giomer restorative material: results at eight years. J Am Dent Assoc. 2007;138(5):621-627.
50. Yap AU, Tham SY, Zhu LY, Lee HK. Short-term fluoride release from various aesthetic restorative materials. Oper Dent. 2002;27(3):259-265.
51. Kouzmina E, Smirnova T, Pazdnikova N. A one-year clinical study of the efficacy of a pit-and-fissure sealant containing bioactive glass. Oral Health and Dental Management in the Black Sea Countries. 2009;8(1):7-12.
52. Skrtic D, Hailer AW, Takagi S, et al. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996; 75(9):1679-1686.
53. Meyer JL, Eanes ED. A thermodynamic analysis of the amorphous to crystalline calcium phosphate transformation. Calcif Tissue Res. 1978;25(1):59-68.
54. Silva KG, Pedrini D, Delbem AC, et al. In situ evaluation of the remineralizing capacity of pit and fissure sealants containing amorphous calcium phosphate and/or fluoride. Acta Odontol Scand. 2010;68(1):11-18.
55. Sharma S, Kugel G. Amorphous calcium phosphate sealants—the potential to remineralize. Inside Dentistry. 2009;5(4):78-80.
56. Gupta K, Tewari A, Sahni A, et al. Remineralizing efficacy of a mineral enriched mouth rinse and fluoridated dentifrice on artificial carious lesions: an in vivo scanning electron microscopic study. J Indian Soc Pedod Prev Dent. 1998;16(3):67-71.
57. Casals E, Boukpessi T, McQueen CM, et al. Anticaries potential of commercial dentifrices as determined by fluoridation and remineralization efficiency. J Contemp Dent Pract. 2007;8(7):1-10.
58. Sano H, Nakashima S, Songpaisan Y, Phantumvanit P. Effect of a xylitol and fluoride containing toothpaste on the remineralization of human enamel in vitro. J Oral Sci. 2007;49(1):67-73.
59. Onishi T, Umemura S, Yanagawa M, et al. Remineralization effects of gum arabic on caries-like enamel lesions. Arch Oral Biol. 2008;53(3):257-260.
60. Leach SA, Lee GT, Edgar WM. Remineralization of artificial caries-like lesions in human enamel in situ by chewing sorbitol gum. J Dent Res. 1989;68(6):1064-1068.
61. Kashket S, Yaskell T, Lopez LR. Prevention of sucrose-induced demineralization of tooth enamel by chewing sorbitol gum. J Dent Res. 1989;68(3):460-462.
62. Manning RH, Edgar WM. Salivary stimulation by chewing gum and its role in the remineralization of caries-like lesions in human enamel in situ. J Clin Dent. 1992;3(3):71-74.
63. Manning RH, Edgar WM, Agalmanyi EA. Effects of chewing gums sweetened using sorbitol or a sorbitol/xylitol mixture on the remineralisation of human enamel lesions in situ. Caries Res. 1992;26(2):104-109.
64. Cai F, Shen P, Walker GD, et al. Remineralization of enamel subsurface lesions by chewing gum with added calcium. J Dent. 2009;37(10):763-768.
65. Reynolds EC, Cai F, Cochrane NJ, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87(4):344-348.
66. Flatt CC, Warren-Morris D, Turner SD, Chan JT. Effects of a stannous fluoride-impregnated dental floss on in vivo salivary fluoride levels. J Dent Hyg. 2008;82(2):19.
67. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31(10):711-718.
68. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247-252.
69. Malhotra N, Mala K, Acharya S. Current strategies and applications of tissue engineering in dentistry—a review. Part 1. Dent Update. 2009;36(9):577-582.
70. Malhotra N, Mala K, Acharya S. Current strategies and applications of tissue engineering in dentistry—a review. Part 2. Dent Update. 2009;36(10):639-646.
71. Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531-535.
72. Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21(9):1025-1032.
73. Balooch G, Marshall GW, Marshall SJ, et al. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J Biomech. 2004;37(8):1223-1232.
74. Roveri N, Battistella E, Bianchi C L, et al. Surface enamel remineralization: Biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomaterials. 2009; Article ID 746383:1-9.
About the Authors
Arathi Rao, MDS
Professor and Head of Department
Department of Pedodontics and Preventive Dentistry
Manipal College of Dental Sciences
Manipal University
Mangalore, India
Neeraj Malhotra, MDS, PGDHHM
Assistant Professor
Department of Conservative Dentistry and Endodontics
Manipal College of Dental Sciences
Manipal University
Mangalore, India